Cariprazine short-term efficacy
CARIPRAZINE SHORT-TERM EFFICACY
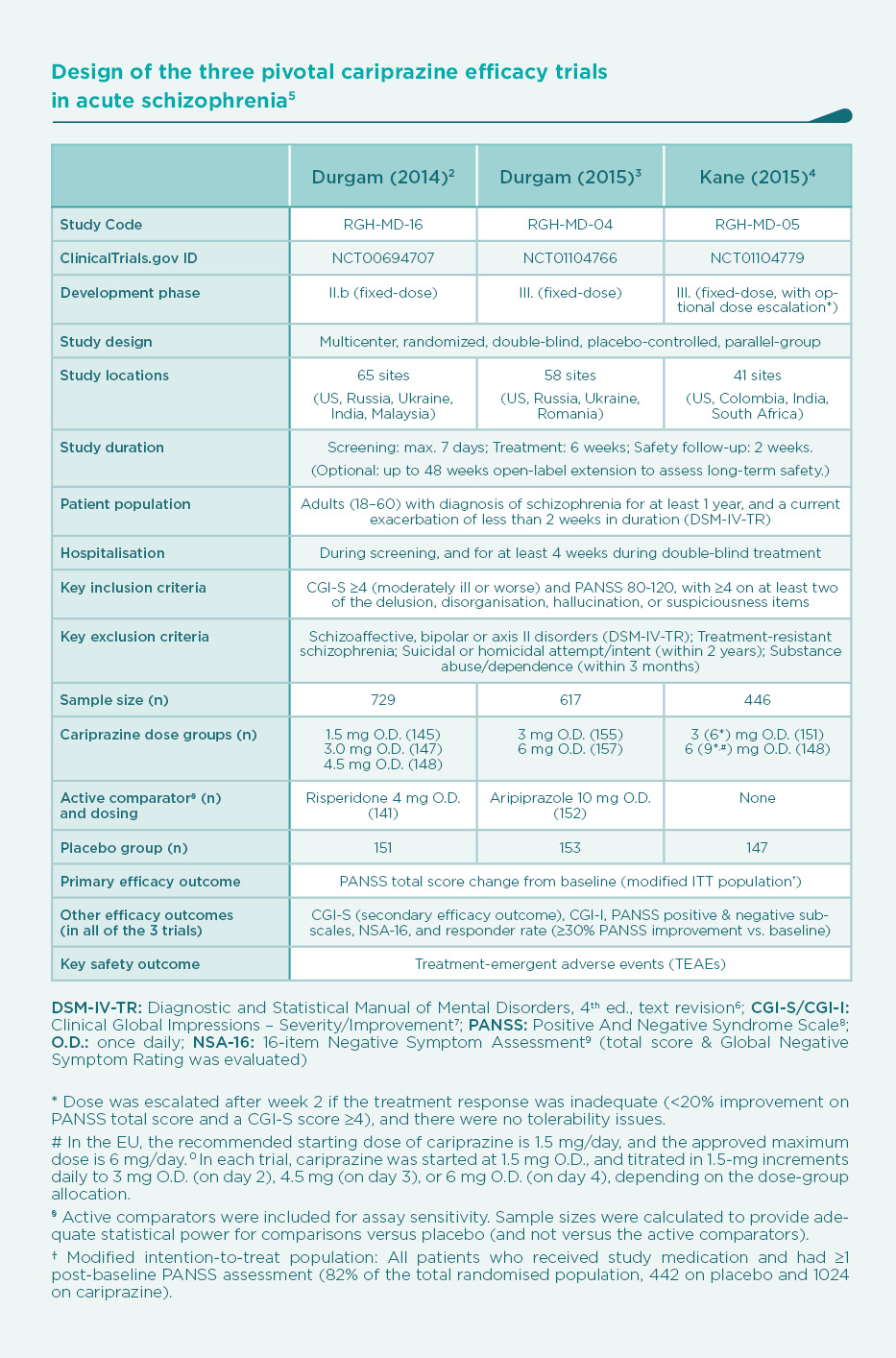
After a proof-of-concept dose-ranging study, three pivotal phase IIb/III, double-blind, randomised trials2,3,4 were conducted to assess the efficacy of cariprazine vs. placebo in patients with acute exacerbation of schizophrenia (Table). In two of the trials, active comparators (risperidone3 or aripiprazole4) were also included for assay sensitivity, but the studies were statistically powered with a sample size for placebo-comparisons only.
Primary efficacy outcome – PANSS total score
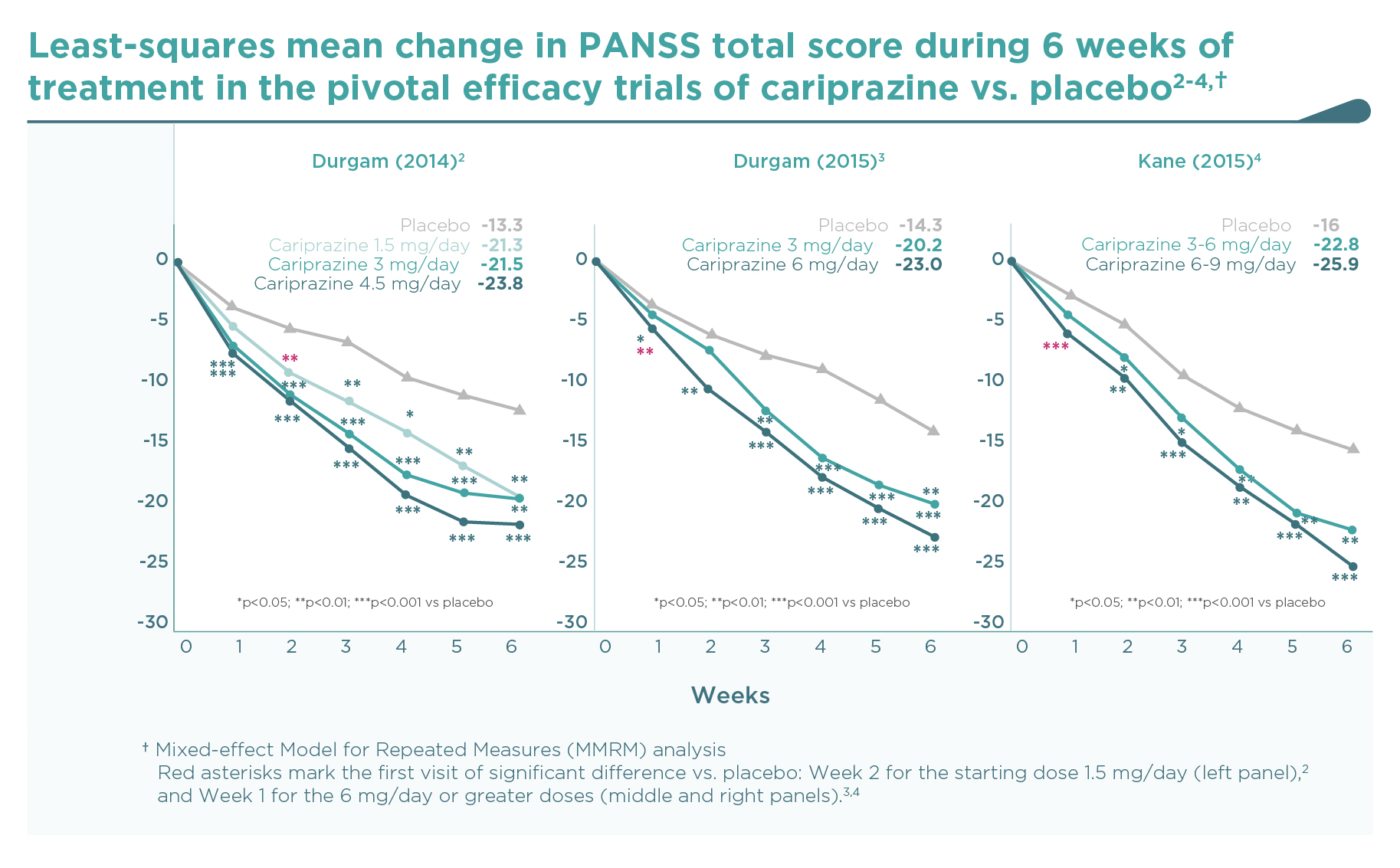
The decrease of PANSS total score from baseline after 6 weeks of treatment is shown on Figure 1. As expected, there was a substantial placebo response, resulting in a continuous decrease of PANSS total score during the 6-week double-blind treatment phase of the trials. Compared to placebo, patients on cariprazine demonstrated a significantly superior and clinically relevant additional decrease in PANSS score over the entire dose range studied, i.e., from 1.5 mg to 9 mg once daily with clear dose-response. Nevertheless, a statistically significant separation of effects from placebo is observed earlier (1 week after randomisation) with the higher doses (4.5 mg/day and above).2-4 But even the lowest dose of cariprazine (1.5 mg/day, i.e., the recommended starting dose) demonstrated continuous, significant improvement in PANSS total score from the second week of treatment, and this superior efficacy was maintained throughout the remainder of the study.2
Secondary efficacy outcome – CGI-S score
The CGI-S score categorizes the severity of the global impression of mental diseases as rated by the expert clinician. Severity categories are as follows: normal subjects are rated 1, borderline 2, mildly ill 3, moderately ill 4, markedly ill 5, severely ill 6, and the extremely ill patients are rated 7.7 Average baseline value in the three pivotal trials was nearly 5.2-4
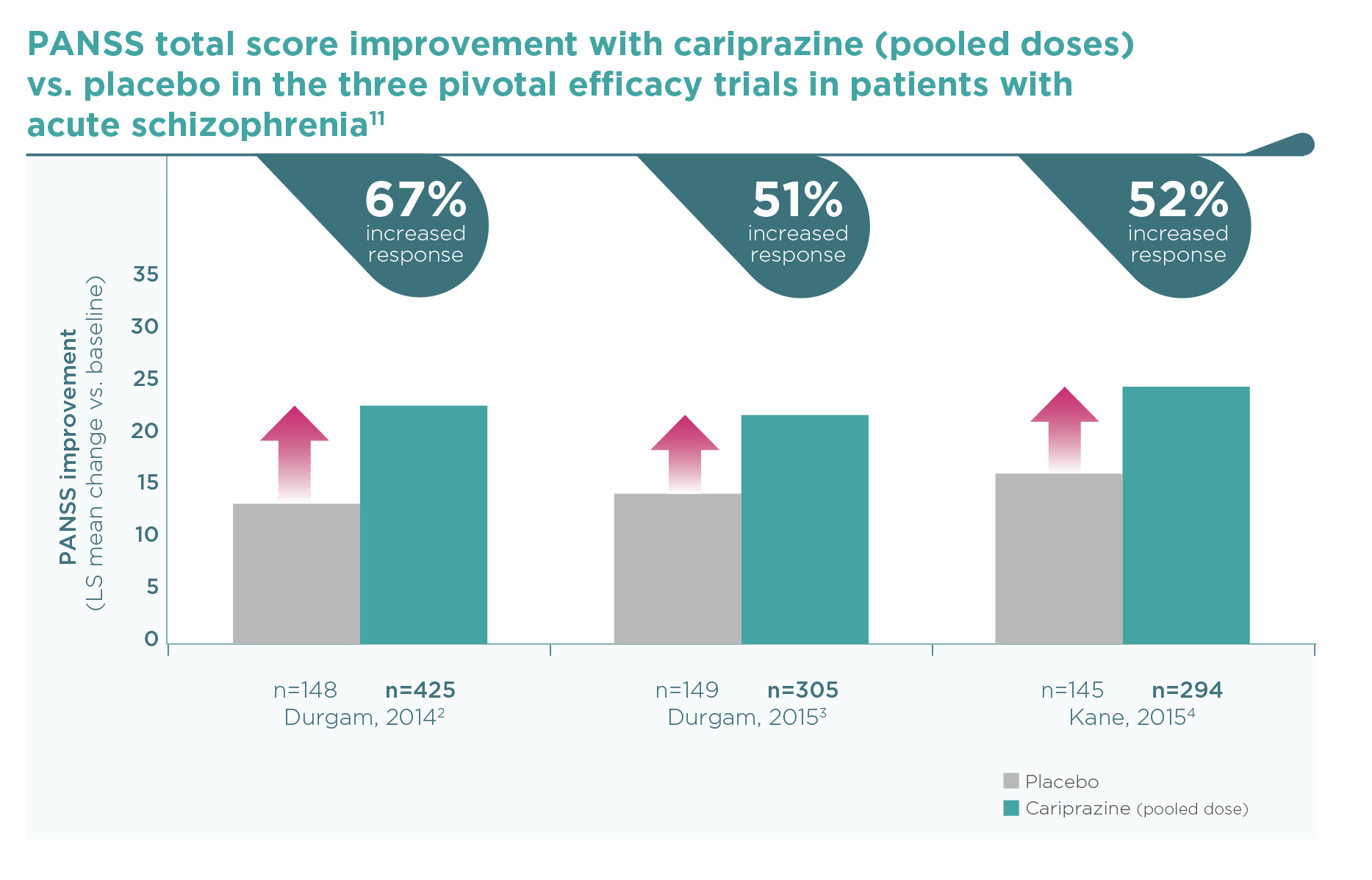
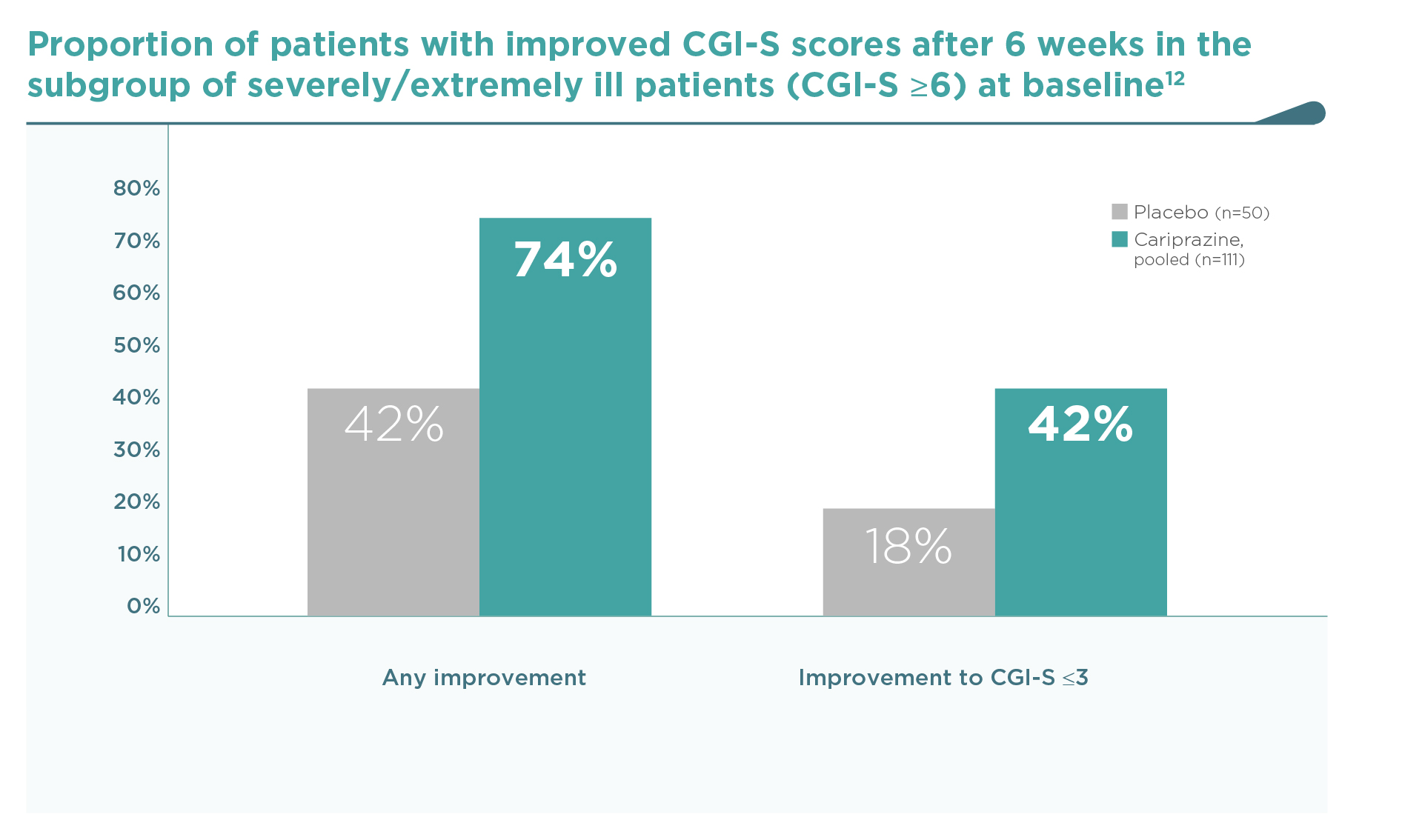
Similarly to the PANSS outcomes, cariprazine was consistently superior to placebo on this key efficacy outcome.2-4 When patients were pooled according to baseline disease severity in a post hoc analysis,12 an increased efficacy was observed in patients with more severe baseline status. In the most severe group of patients with a CGI-S ≥6 at baseline (severely or extremely ill, N=161), response rates were especially high (74% any improvement in CGI-S), even when compared to the high placebo response rate (42% any improvement). A clinically remarkable shift from CGI-S ≥6 (severely or extremely ill) to CGI-S ≤3 (mildly ill, borderline or normal) occurred in a significantly greater proportion with cariprazine vs. placebo (42% vs. 18%, P<0.01) (Figure 3).
In the subgroup of severely/extremely ill patients (CGI-S ≥6, N=161) at baseline…
Cariprazine reduced disease severity in 74% (vs. 42% on placebo), and
42% of them shifted from the severely/extremely ill category to the mildly
ill, borderline or normal state (CGI-S ≤3) (vs. 18% on placebo, p<0.01).12
Safety outcomes in the short-term efficacy trials
A post-hoc safety/tolerability analysis5 in patients with acute exacerbations of schizophrenia was performed using the data of the four phase II/III trials.1-4 Safety population was defined as all patients who received ≥1 dose of study drug. Subgroups according to categories of modal daily dose (which is defined as the most frequently administered dose per patient) were separately analysed: 1.5–3 mg/day, 4.5–6 mg/day, and 9–12 mg/day. Cariprazine was generally well tolerated.
The incidence of treatment-emergent adverse events (TEAE) versus placebo was similar for cariprazine 1.5–3 mg/day and higher for cariprazine 4.5–6 and 9–12 mg/day. There was a dose–response relationship for akathisia, extrapyramidal symptoms, and diastolic blood pressure as well as some laboratory parameters (creatine phosphokinase [CPK] and transaminase elevations). Cariprazine was associated with small (∼1 to 2 kg) increases in body weight vs. placebo, but no difference was observed in metabolic parameters, prolactin level, or QTc prolongation (>500 ms). Within the 1.5-6.0 mg/day dose range approved by the EU (EMA) and US (FDA) regulatory agencies, cariprazine was generally safe and well tolerated in patients with schizophrenia.5
References
2 Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. https://www.ncbi.nlm.nih.gov/pubmed/24412468
3 Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Németh G, Meltzer HY. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015 Dec; 76(12):e1574-1582. https://www.ncbi.nlm.nih.gov/pubmed/26717533
4 Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results From an International, Phase III Clinical Trial. J Clin Psychopharmacol. 2015 Aug; 35(4):367-373. https://www.ncbi.nlm.nih.gov/pubmed/26075487
5 Earley W, Durgam S, Lu K, et al. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: a pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int Clin Psychopharmacol 2017; 32:319–328. https://www.ncbi.nlm.nih.gov/pubmed/28692485
6 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association, 2000.
7 Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: publication ADM. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch, 1976:76–338.
8 Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
9 Axelrod BN, Goldman RS, Alph LD. Validation of the 16-item Negative Symptom Assessment. J Psychiatr Res. 1993;27(3):253–258.
10 REAGILA (cariprazine) Summary of Product Characteristics. European Medicines Agency, auth. nr. EU/1/17/1209. http://ec.europa.eu/health/documents/community-register/html/h1209.htm
11 Zhao MJ, et al. Efficacy and acceptability of cariprazine in acute exacerbation of schizophrenia: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2018;38(1):55-59.
12 Durgam S, Earley W, Lu K, Németh G, Laszlovszky I, Volk S, Litman RE. Global improvement with cariprazine in the treatment of bipolar I disorder and schizophrenia: A pooled post hoc analysis. Int J Clin Pract. 2017 Dec;71(12). https://www.ncbi.nlm.nih.gov/pubmed/29119668




