Cariprazine long-term safety
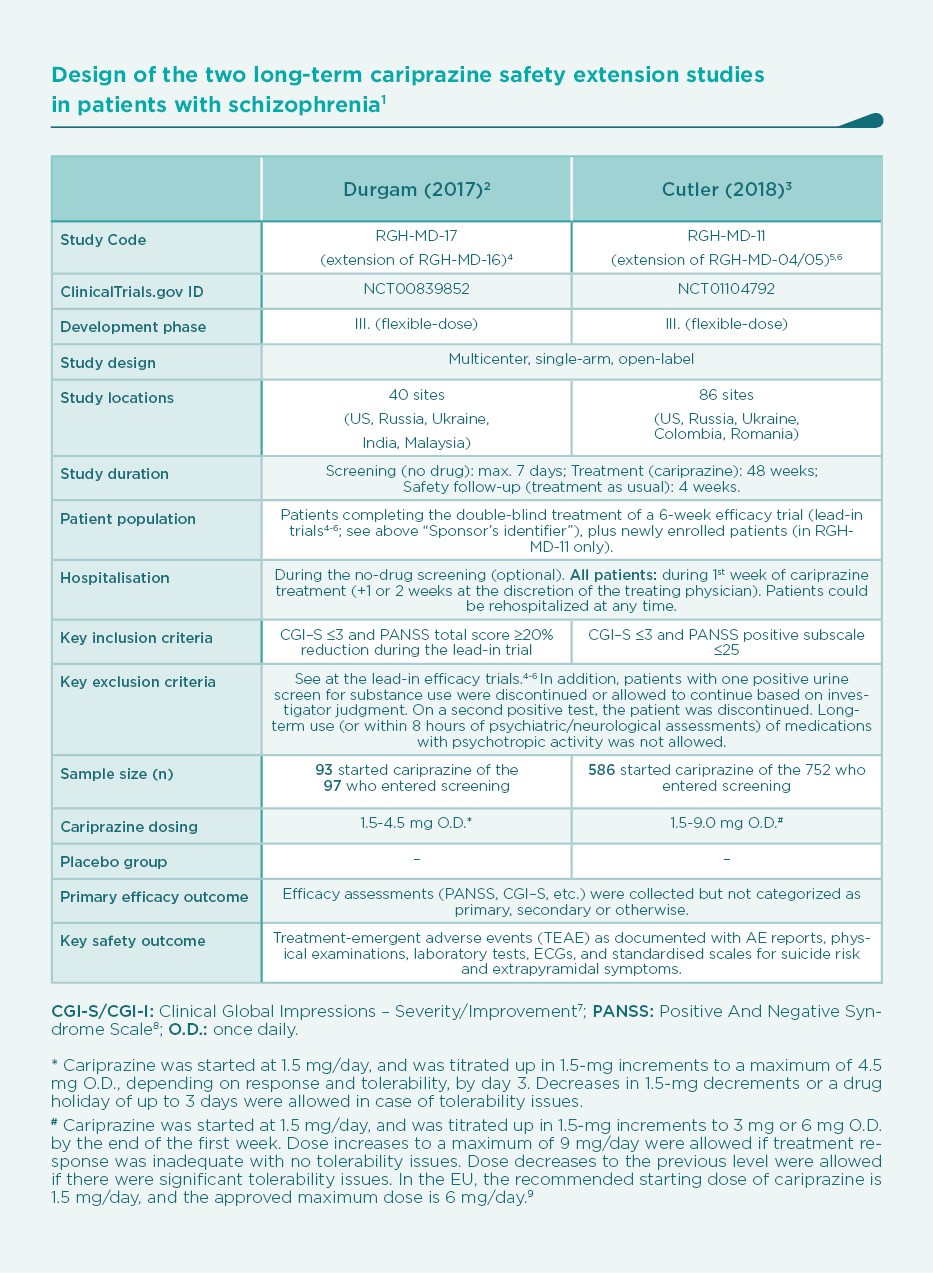
The second 48-week, open-label extension study (RGH-MD-11) evaluated the long-term safety and tolerability of cariprazine at higher doses
(3–9 mg/day). The mean treatment duration was 183 days with a modal daily dose (which is defined as the most frequently administered dose per patient) of 9.0 mg/day (25.3% of patients), 6.0 mg/day (50.9%), 3.0 mg/day (22.9%), or 1.5 mg/day (1.0%).3
In order to assess the potential dose-dependence of adverse events and to closely evaluate the safety profile of the approved dose range (1.5 to 6.0 mg/day), data from the two extension studies were pooled, and examined according to the following modal daily dose categories: 1.5–3 mg, 4.5–6 mg, and 9 mg cariprazine once daily.1
Study population (pooled)
The pooled safety population consisted of 679 patients, of whom 416 had not previously been exposed to cariprazine (received placebo or an active comparator in a lead-in study, or newly enrolled into RGH-MD-11). The total cariprazine exposure amounted to 350 patient-years. Mean age was 38.5 years, with male predominance (69.4%), and a mean duration of schizophrenia of 12.2 years (age at onset: 26.2).17 Almost 80% of patients were in the approved dose range (1.5–6 mg/day) (Table 4).1
The baseline value for safety parameters was the last value measured before the first dose of the study drug (cariprazine, placebo or an active control). Mean changes in safety parameters were evaluated from baseline to end of study, i.e., the last available assessment during the open-label treatment. Safety assessments occurred weekly during the first 6 weeks of open-label cariprazine treatment, and biweekly thereafter.2,3
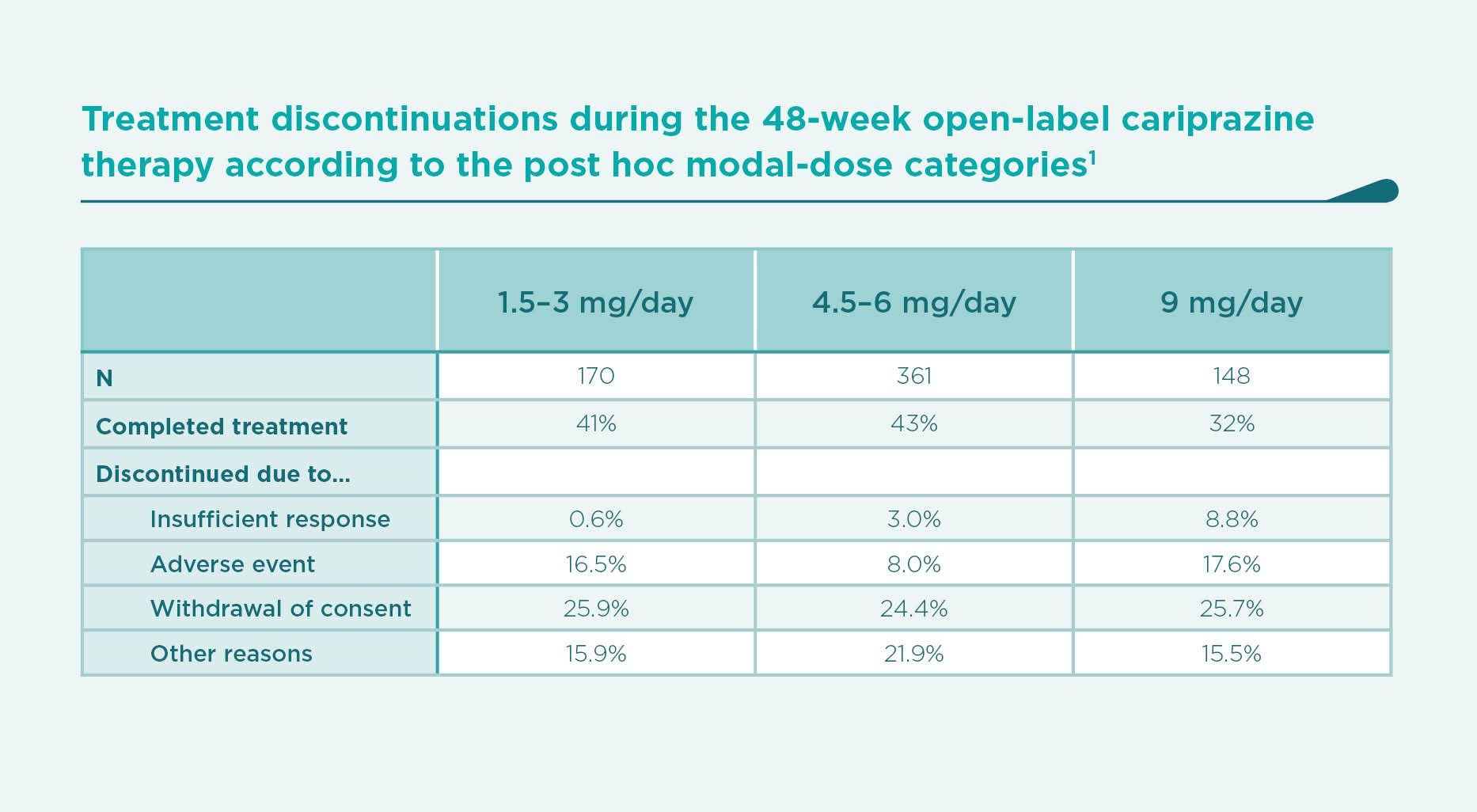
Discontinuations: A textbook example of confounding by indication
In Table 4, the chance of discontinuing therapy due to insufficient response appears to be paradoxically proportional to the increasing modal daily dose.1 It has to be highlighted that the post hoc categorisation of patients does not constitute a randomised comparison of various dose groups. In the group of patients who needed to be titrated up to 9 mg/day cariprazine, poor responders are over-represented in flexible-dose studies like the present ones. The high-dose group is therefore at an elevated risk of discontinuation due to insufficient response. This, in turn, affects their persistence on therapy, their chances of exhibiting certain time-dependent side effects, and discontinuing due to adverse events (see the lowest completion rate in the 9-mg dose group in Table 2).
The same paradoxical association applies for the high rates of discontinuation due to adverse events among those treated with the lowest doses (compared to the medium dose group).1 Similarly as above, patients with tolerability issues are over-represented in the lowest-dose group, which in fact caused them to be down-titrated to the lowest doses.
Obviously, it is not the lowest dose which causes the high rate of adverse events, and it is not the highest dose which causes the high rate of insufficient response. Rather on the contrary: poor response and tolerability issues constitute an indication of up-titration and down-titration, respectively, and are the very causes of discontinuations due to inefficacy and intolerance, respectively. The bias of this kind is called “confounding by indcation”.10 Any comparisons across the dose groups must be interpreted with these caveats in mind.1
Treatment-emergent adverse events (TEAEs)
Akathisia, insomnia, headache, and weight increased were the most common TEAEs, reported in 10-15% of patients each. EPS-related TEAEs other than akathisia/restlessness were reported in 19.9% of patients (mostly as tremor and “extrapyramidal disorder”). Tardive dyskinesia was reported in 2 patients. Almost 98% of EPS-related AEs, including akathisia/restlessness, were considered mild or moderate. The majority (80%) of akathisia TEAEs occurred in the first 6 weeks of treatment, and the rest beween weeks 7-48. Although akathisia showed an inverse dose-response relationship, this is most likely explained with confounding by indication (see in the Discontinuation section above).1,10 In the safety analysis of the pooled short-term efficacy trials, incidence of akathisia was directly proportional to the dose levels.11
Similarly due to confounding by indication, schizophrenia adverse event was spuriously reported most commonly (10.8%) in the 9-mg dose group. Anxiety and tremor were reported in 10% of patients in the medium and lowest dose groups, respectively. Somnolence and sedation were reported in 3.8% and 3.1% of patients, respectively.1
The only adverse events (AEs) that led to discontinuation of ≥2% of patients in any dose group were akathisia, worsening of schizophrenia, and psychotic disorder. Of patients with TEAEs that resulted in discontinuation, 80.3% had events that resolved, in a median period of 12 days (15 days for akathisia/restlessness) after discontinuing cariprazine. These results confirm that there was no evidence of delayed resolution of adverse effects after cariprazine discontinuation.1
AEs that resulted in discontinuation resolved in 80% of patients, with the median time to resolution of 12 days (15 days for akathisia/restlessness).1
Laboratory and cardiometabolic parameters
Serum chemistry and hematology parameters did not change in a clinically meaningful degree. Mean prolactin levels decreased in all dose groups. There was a minimal increase in aminotransferase (AST, ALT) levels, and a minimal decrease in alkaline phosphatase (AP). Two patients were discontinued due to increased aminotransferase levels. Creatine kinase (CPK) levels showed a dose-dependent increase, similarly to the short-term efficacy trials. There were no mean increases in lipid parameters, however, the incidence of significant treatment-emergent changes in fasting glucose tended to increase over time. Mean weight change from baseline was 1.58 kg, and there was no dose-response. There was no mean increase in QTc interval overall; 3 patients had a postbaseline QTcB value >500 msec. Hypertension AEs occurred more commonly in the 9-mg dose category (8.7%) vs. the other groups (approx. 3% each).1
Serious adverse events (SAEs)
The most commonly reported SAEs: worsening of schizophrenia (4.4%) and psychotic disorder (2.1%). Due to confounding by indication (see in the Discontinuation section above), these events occurred most commonly among patients in the highest-dose group (6.8% for worsening schizophrenia, and 4.7% for psychotic disorder).1
There were no SAEs of akathisia, restlessness, or EPS. The same applies for ocular events, and it can be stated that ophthalmologic AEs associated with cariprazine appear to be rare and not likely related to treatment.1
In the study RGH-MD-11, treatment-emergent suicidal ideation was found in 2.8% of patients on the C-SSRS tool. Suicidal behaviour was not noted. In RGH-MD-17, TEAEs of suicidal ideation were reported in 4 (0.6%) patients, but mean STS scores did not change. Among the 7 patients with non-zero STS total scores, there were no TEAEs related to suicidal ideation or suicidal behaviour. Over the 350 patient-years of cariprazine therapy, one patient completed suicide in the 4.5–6 mg/day dose group; the event was not considered related to cariprazine.1
Conclusion
In this post hoc pooled analysis of data from 2 long-term open-label studies, there was no new safety concern related to the 1-week effective half-life of cariprazine with its active metabolites. Treatment with cariprazine was generally safe and well tolerated. These results support the safety and tolerability of cariprazine within the dose range of 1.5–6 mg/day approved by both the FDA and EMA for the treatment of schizophrenia.1
REFERENCES
2 Durgam S, Greenberg WM, Li D, Lu K, Laszlovszky I, Nemeth G, Migliore R, Volk S4 Safety and tolerability of cariprazine in the long-term treatment of schizophrenia: results from a 48-week, single-arm, open-label extension study. Psychopharmacology. 2017;234(2):199–209. https://www.ncbi.nlm.nih.gov/pubmed/27807604
3 Cutler AJ, Durgam S, Wang Y, Migliore R, Lu K, Laszlovszky I, Németh G. Evaluation of the long-term safety and tolerability of cariprazine in patients with schizophrenia: results from a 1-year open-label study. CNS Spectr. 2018;23:39–50. https://www.ncbi.nlm.nih.gov/pubmed/28478771
4 Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. https://www.ncbi.nlm.nih.gov/pubmed/24412468
5 Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Németh G, Meltzer HY. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015 Dec; 76(12):e1574-1582. https://www.ncbi.nlm.nih.gov/pubmed/26717533
6 Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results From an International, Phase III Clinical Trial. J Clin Psychopharmacol. 2015 Aug; 35(4):367-373. https://www.ncbi.nlm.nih.gov/pubmed/26075487
7 Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: publication ADM. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch, 1976:76–338.
8 Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
9 REAGILA (cariprazine) Summary of Product Characteristics. European Medicines Agency, auth. nr. EU/1/17/1209. http://ec.europa.eu/health/documents/community-register/html/h1209.htm
10 Catalogue of bias collaboration, Aronson JK, Bankhead C, Mahtani KR, Nunan D. Confounding by indication. In Catalogue Of Biases, Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford, 2018. https://catalogofbias.org/biases/confounding-by-indication/
11 Earley W, Durgam S, Lu K, et al. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: a pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int Clin Psychopharmacol 2017; 32:319–328. https://www.ncbi.nlm.nih.gov/pubmed/28692485




