Cariprazine efficacy on negative symptoms
Drug efficacy on negative symptoms: Depends on the patient population
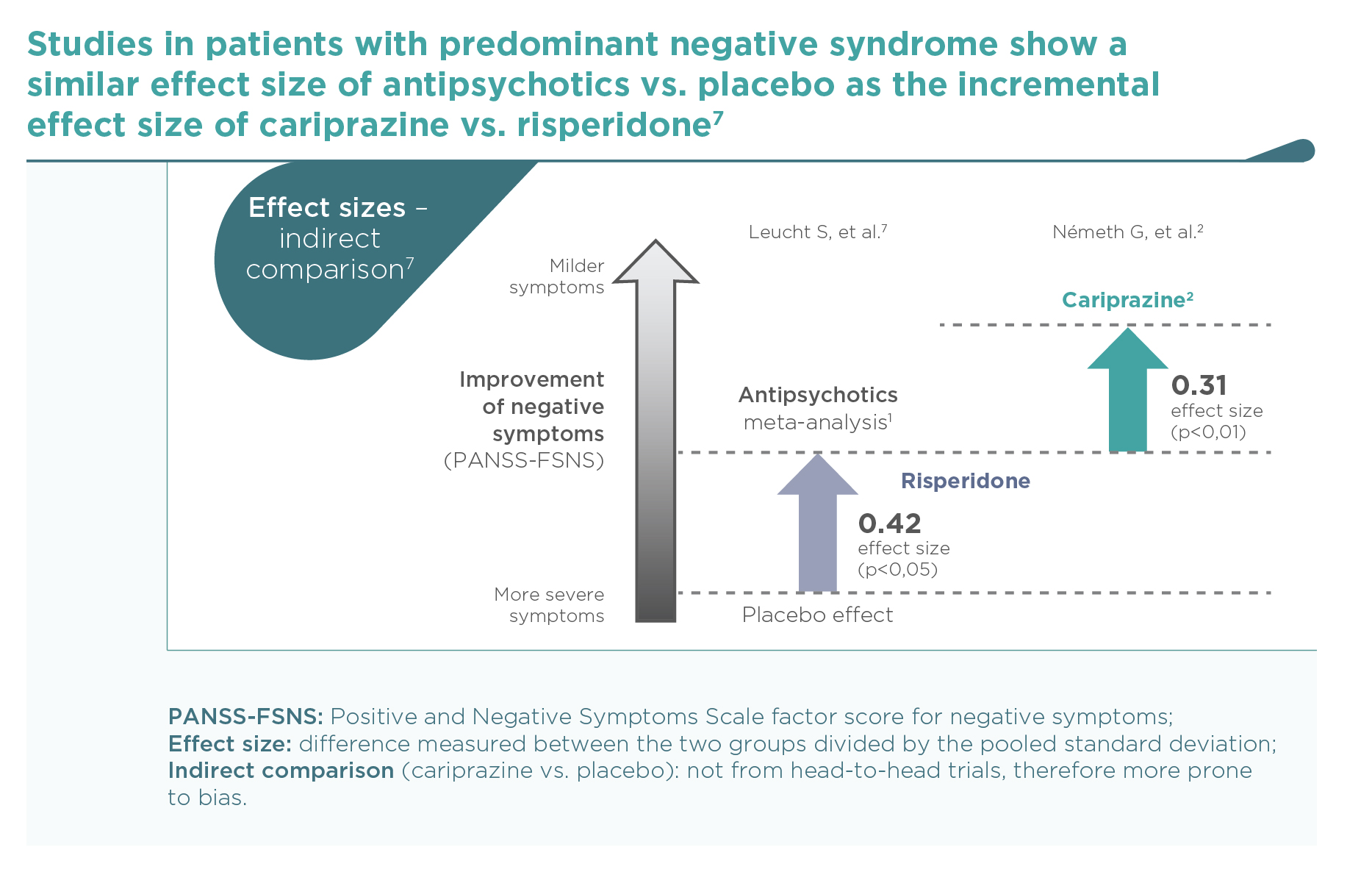
Current knowledge supports the possibility that improvements of negative symptoms seen with various first- and second-generation antipsychotics are only secondary to reductions of positive symptoms (which may occupy patients so much that severely limits their volition and social interactions). The difference between some of the agents may be due in part to differences in their extrapyramidal side-effects (which can mimic negative symptoms) or their anti-depressant activities (since depressive and negative symptoms overlap). Possible confounding due to parallel effects on depression, positive symptoms or EPS occurred in many studies of antipsychotics, albeit more frequently in prominent vs. predominant negative symptoms studies.1
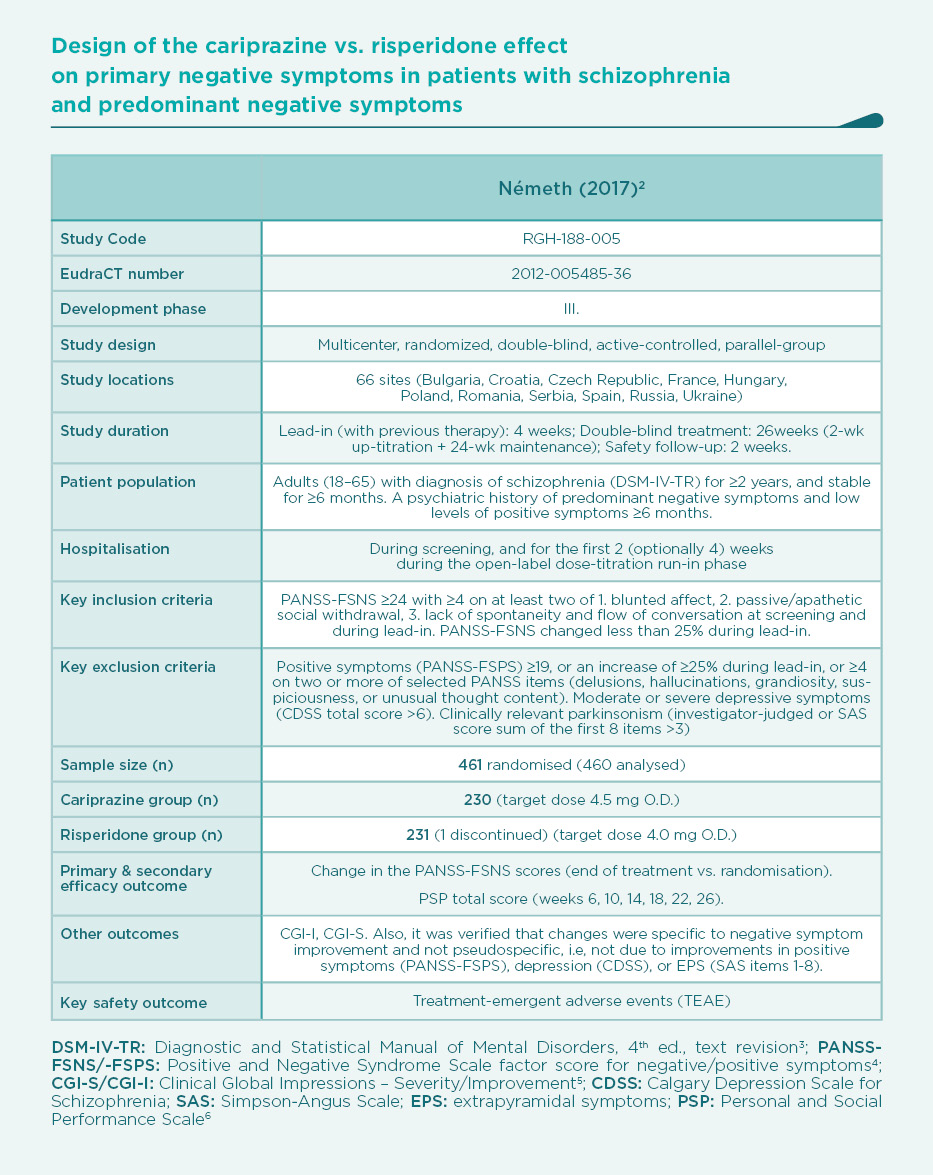
Prominent negative symptoms mean a high degree of negative symptoms, whereas predominant negative symptoms should also meet the additional criteria of no-to-little positive symptoms. The European Medical Agency (EMA) requires predominant negative symptoms in studies that are aimed at demonstrating a true effect on primary negative symptoms. Therefore, the cariprazine vs. risperidone trial was planned according to EMA’s stringent criteria (see Table).2
Results
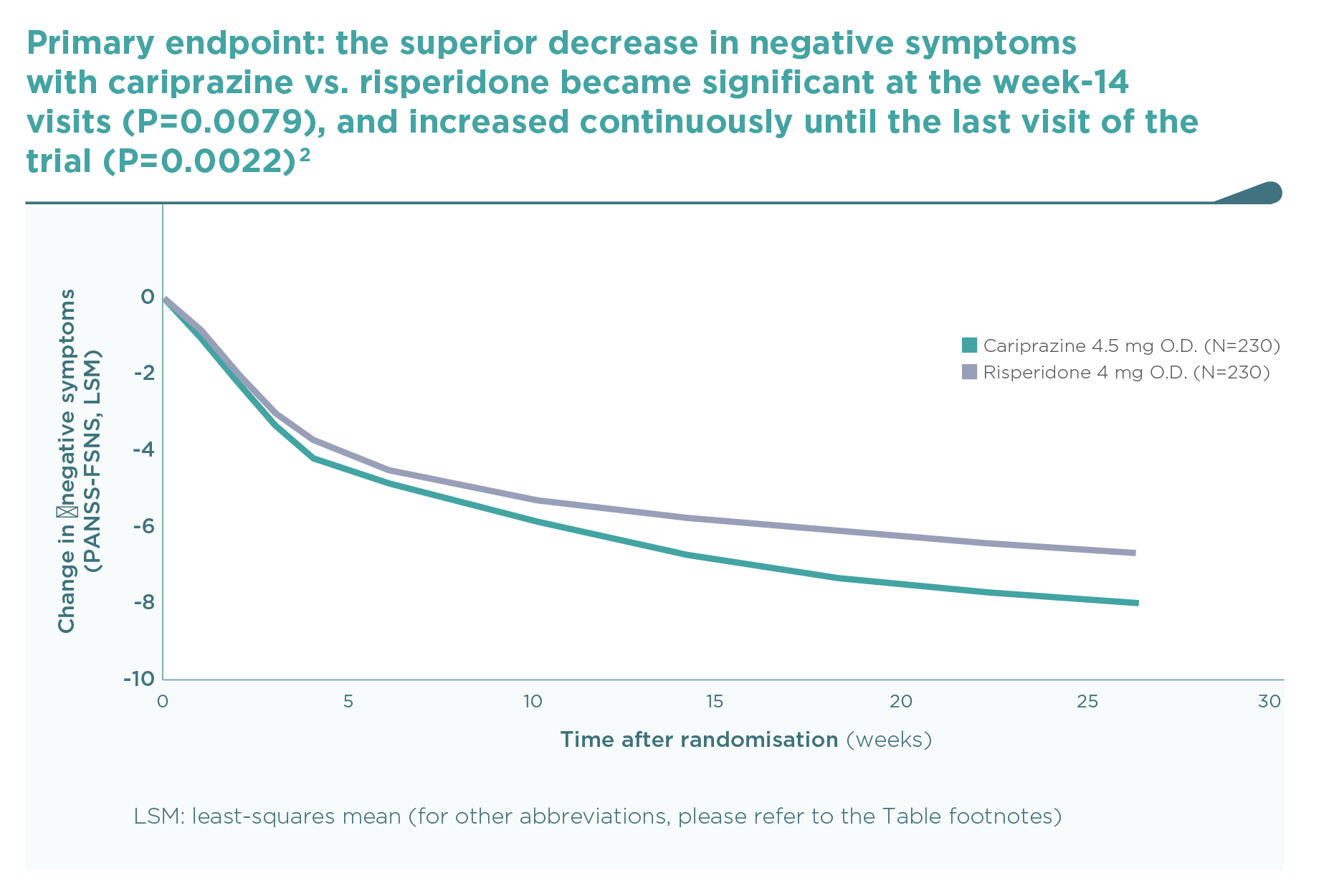
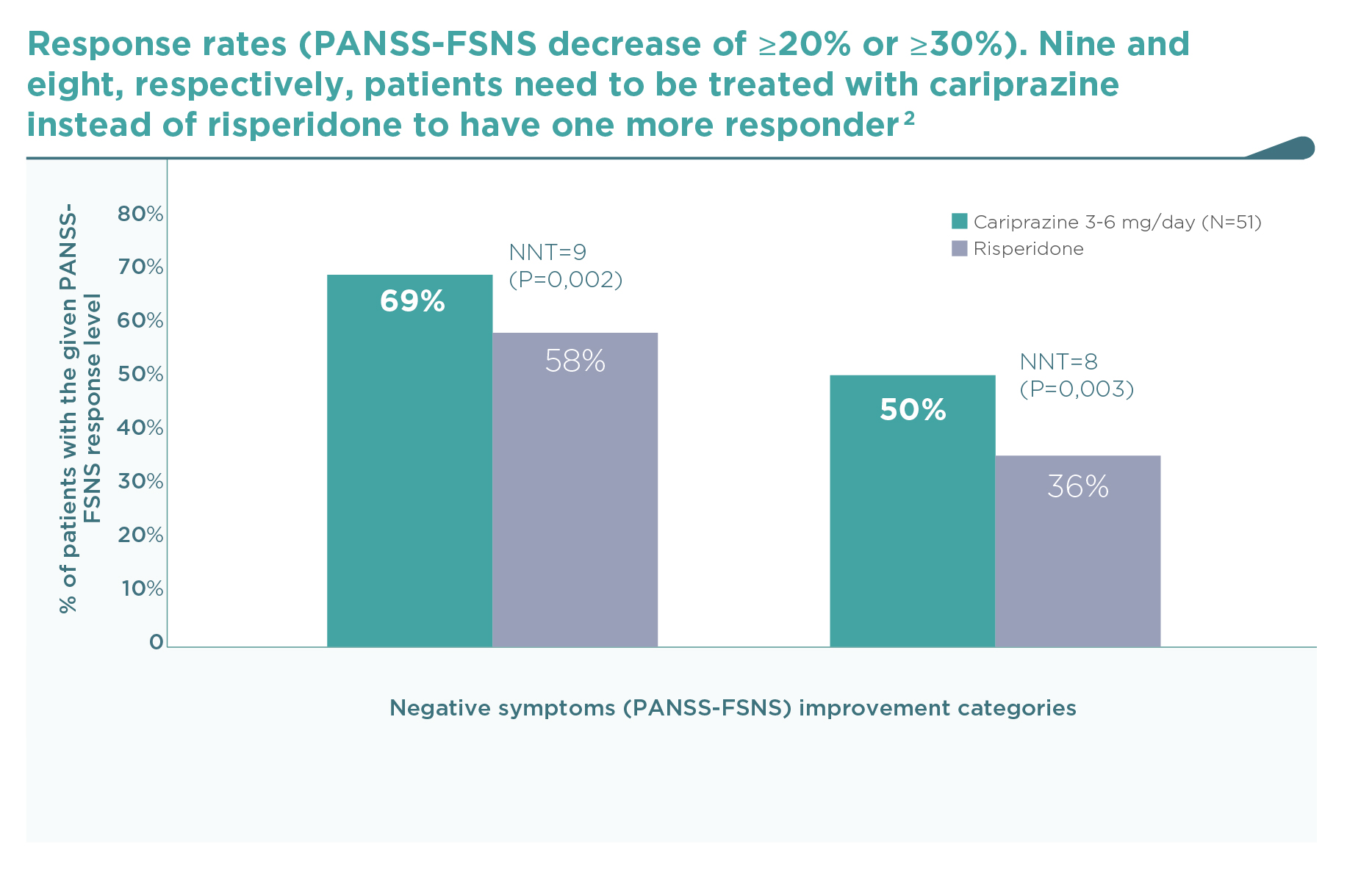
This was the first large trial in patients with predominant negative symptoms, in which drug effects on positive symptoms, depressive symptoms, and extrapyramidal side-effects were controlled: in fact, there were minimal effects of either cariprazine or risperidone in terms of positive (∆PANSS-FSPS), depressive (∆CDSS), or extrapyramidal symptoms (∆SAS items 1-8) compared to the patients’ stable treatment received before randomisation, and there was no difference between the two groups (P>0.5 for each comparison). The entry criteria of well-controlled positive symptoms (baseline PANSS-FSPS score 8.6-8.7) ensured that the switch to either of the randomised therapies did not result in a material effect. This is in contrast to the effect detectable on the severe negative symptoms (baseline PANSS-FSNS score 27.6). The use of cariprazine led to a greater least squares mean (LSM) change from baseline to week 26 in PANSS-FSNS than did risperidone (–8.90 vs. –7.44; difference –1.46, 95% CI –2·39 to –0·53; p=0.0022; effect size=0.31). The superior effect of cariprazine vs. risperidone occurred after the week-14 measurement, and continuously increased up until the end of the trial, i.e., week 26 (Figure 1).2 This time course is different from studies in patients with acute positive symptoms in which improvements usually occur in the first week but also tend to plateau earlier. The different time course suggests different mechanisms, too.1 Greater affinity to D3 than D2 dopamine receptors in addition to preferential limbic action has been discussed as potential mechanisms of action.1,2 This is reflected in the response rates (PANSS-FSNS decrease of ≥20% or ≥30%), and numbers needed to treat to have one additional responder (Figure 2).2
The results of this trial support the efficacy of cariprazine in the treatment of predominant negative symptoms of schizophrenia.
References
2 Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, Barabássy Á, Debelle M, Durgam S, Bitter I, Marder S, Fleischhacker WW. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 2017;389:1103-13.
https://www.ncbi.nlm.nih.gov/pubmed/28185672
3 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association, 2000.
4 Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
5 Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: publication ADM. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch, 1976:76–338.
6 Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329.
7 Leucht S, Davis JM. Schizophrenia, primary negative symptoms, and soft outcomes in psychiatry (Comment). Lancet 2017;389:1077-1078.




