Cariprazine efficacy in relapse prevention
The efficacy of cariprazine for maintaining antipsychotic effect was investigated in a randomized withdrawal, long-term clinical study (Table).1
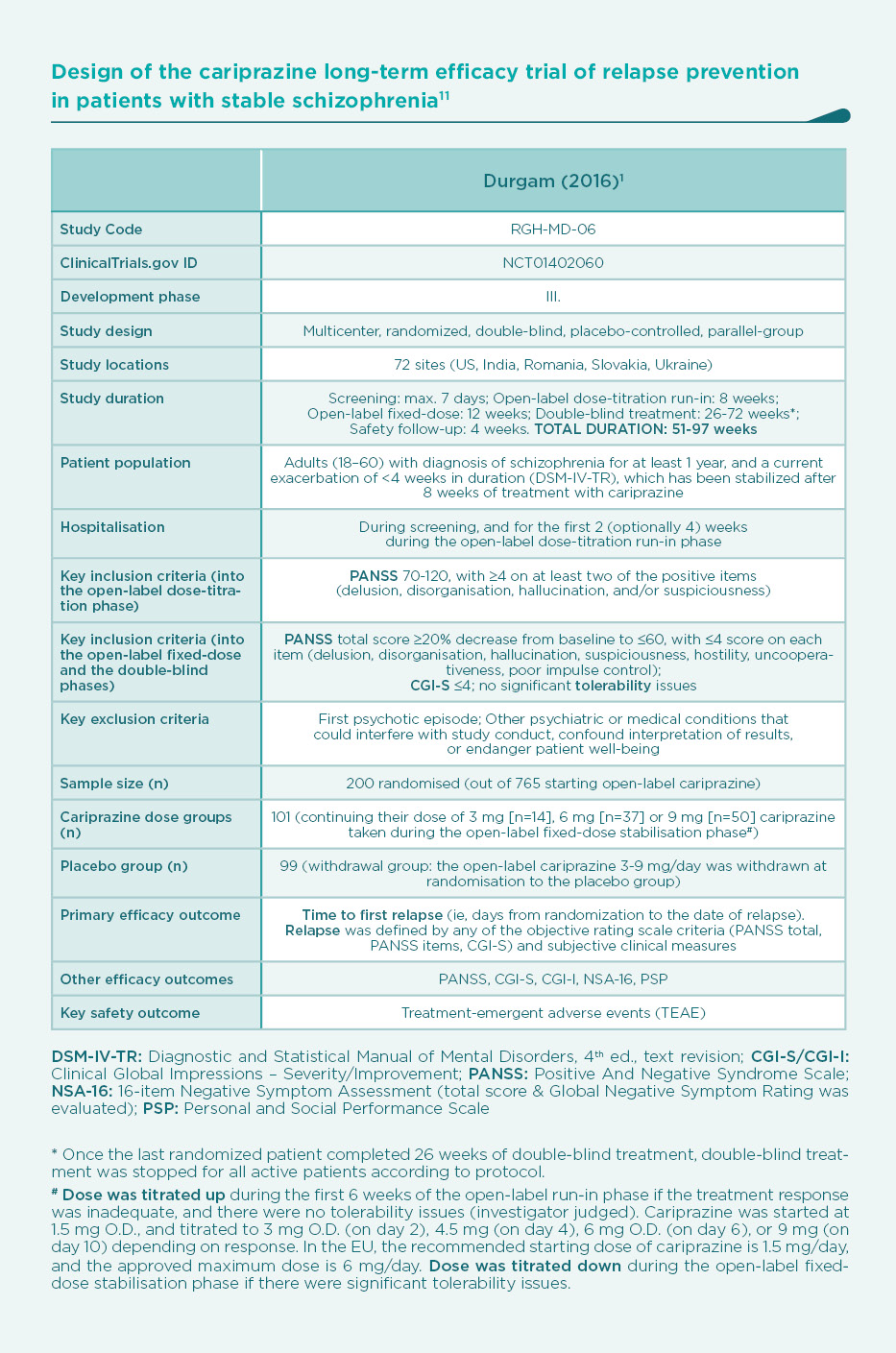
Study population
In total, 751 patients with acute symptoms of schizophrenia received cariprazine 3 9 mg/day for 20 weeks, of whom 337 received cariprazine in the dose-range of 3 or 6 mg/day (based on a post hoc review of the approved doses requested by the European Medicines Agency [EMA]).7,8 Stabilized patients were then randomised to receive fixed doses of 3 or 6 mg/day cariprazine (N=51) or placebo (N=51) in a double-blind manner for up to 72 weeks (minimum of 26 weeks). The primary outcome of the study was time to relapse after randomisation with continuing vs. withdrawing cariprazine therapy.
Primary efficacy outcome – Time to relapse
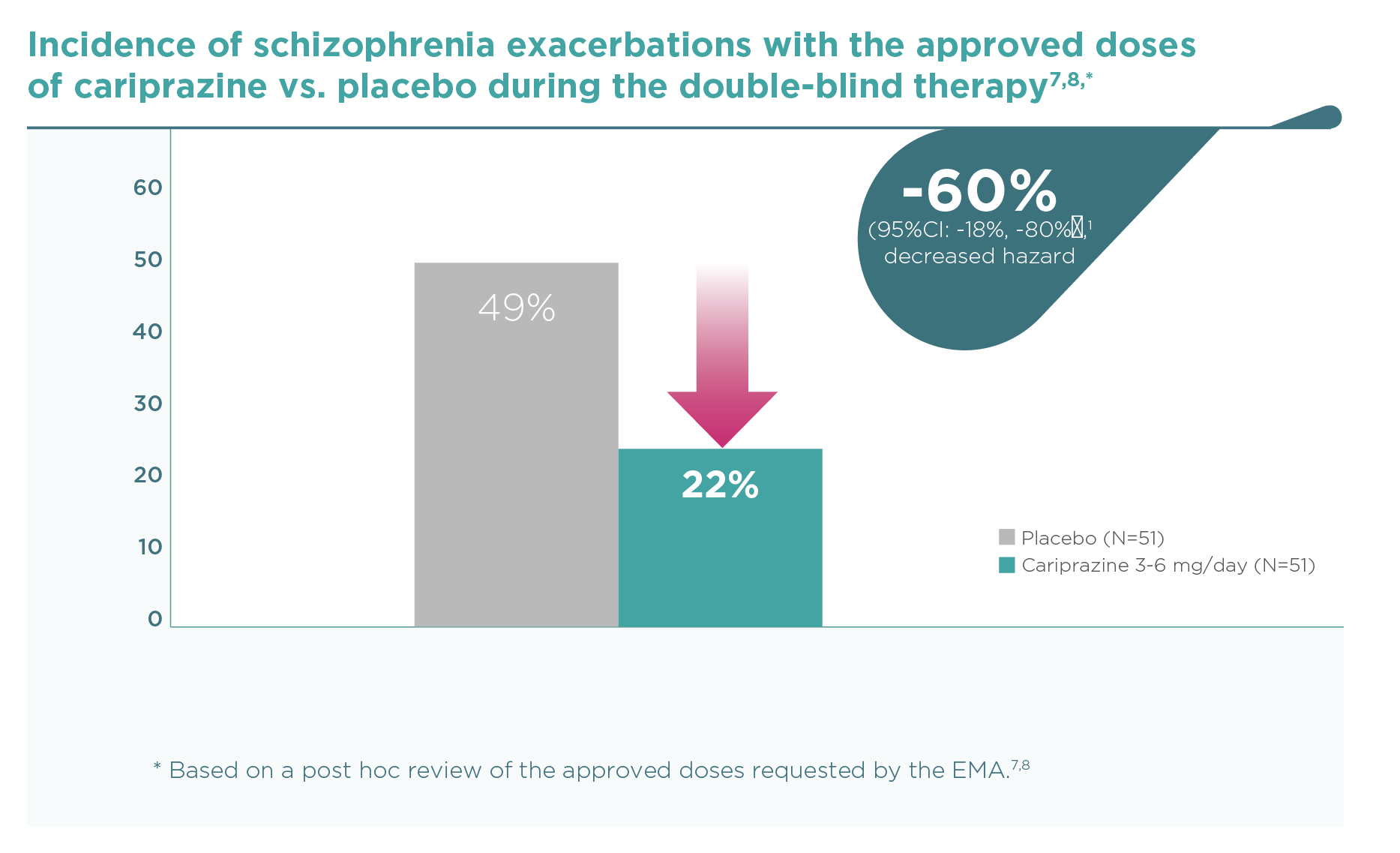
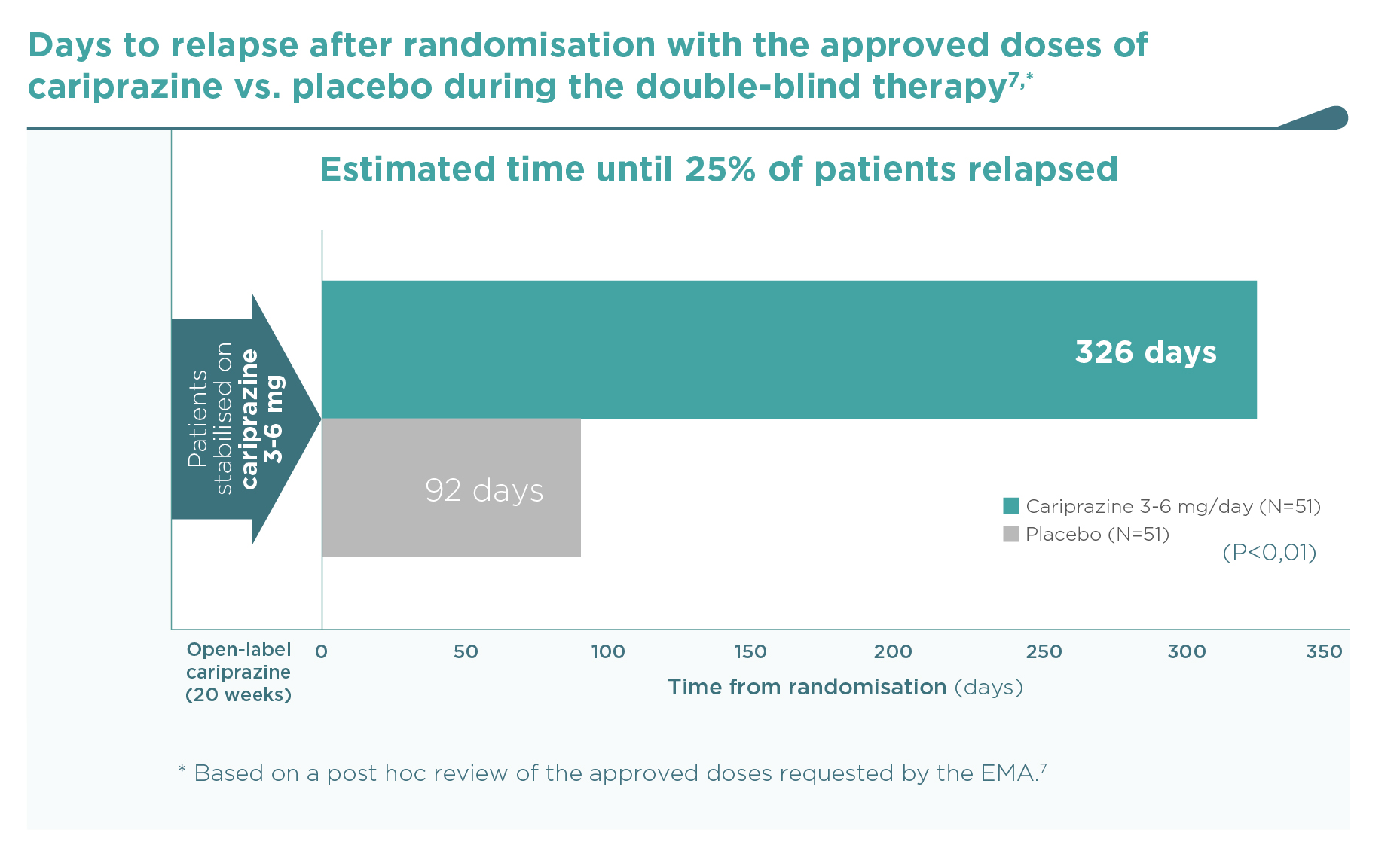
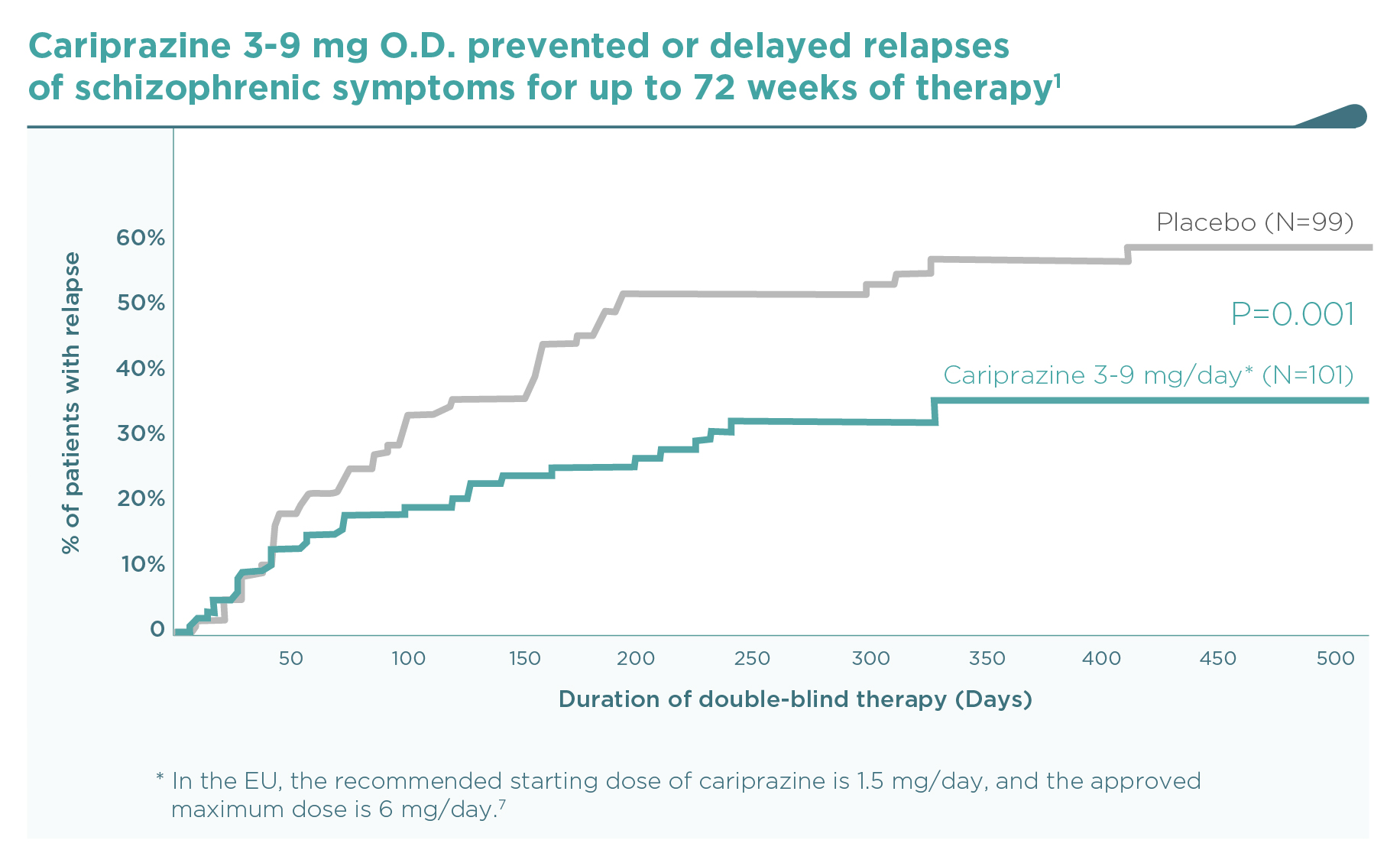
By the end of the trial, 49.0% of placebo-treated patients versus 21.6% of cariprazine-treated patients (3-6 mg/day dose group – post hoc review of the approved doses requested by the EMA) had a relapse of schizophrenic symptoms (hazard ratio [HR] 0.40, 95%CI 0.20, 0.82 – Figure 1).7,8 Time to relapse was therefore significantly longer in the cariprazine group than in the placebo group (92 vs. 326 days based on the 25th percentile, p=0.009 – Figure 2).7. Outcomes for the whole randomised population treated with 3-9 mg/day cariprazine (N=101) vs. placebo (N=99) were similar (Figure 3): HR 0.45 (95%CI 0.28, 0.73); time to relapse in 25% of the population 92 vs. 224 days (P=0.004).1 Five patients needed to be treated with cariprazine 3-9 mg/day instead of placebo in order to prevent one schizophrenia relapse during the 26 to 72 weeks’ double-blind period (NNT=5).1
In patients with schizophrenia stabilised on cariprazine 3-6 mg/day, continued treatment for up to 72 weeks decreased the risk of relapse by 60% (P<0.01 vs. placebo).7,8
Other efficacy outcomes – PANSS, CGI-S, CGI-I, NSA-16, PSP
(For abbreviations, please refer to footnote at Table.) Mean scores on additional efficacy parameters suggested symptom improvement for patients taking open-label cariprazine, and, after randomisation, less worsening of symptoms for double-blind cariprazine-treated patients than for placebo-treated patients.1
Safety outcomes in the long-term efficacy trial
The long-term safety profile of cariprazine was consistent with that observed in the 6-week clinical trials.9,10,11 As in other relapse prevention studies, the population entering the double-blind phase had already been stabilized on cariprazine without significant safety or tolerability issues. Therefore, patients with unacceptable adverse event (AE) or tolerability problems were not part of the double-blind analyses, and the double-blind AE profile should be interpreted with caution.1
References
2 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association, 2000.
3 Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: publication ADM. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch, 1976:76–338.
4 Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
5 Axelrod BN, Goldman RS, Alph LD. Validation of the 16-item Negative Symptom Assessment. J Psychiatr Res. 1993;27(3):253–258.
6 Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329.
7 REAGILA (cariprazine) Summary of Product Characteristics. European Medicines Agency, auth. nr. EU/1/17/1209. http://ec.europa.eu/health/documents/community-register/html/h1209.htm
8 European Medicies Agency (EMA) Committee for Medicinal Products for Human Use (CHMP). Reagila Assessment Report (EPAR), EMA/CHMP/353055/2017, 18 May 2017: p. 113.
9 Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. https://www.ncbi.nlm.nih.gov/pubmed/24412468
10 Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Németh G, Meltzer HY. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015 Dec; 76(12):e1574-1582. https://www.ncbi.nlm.nih.gov/pubmed/26717533
11 Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results From an International, Phase III Clinical Trial. J Clin Psychopharmacol. 2015 Aug; 35(4):367-373. https://www.ncbi.nlm.nih.gov/pubmed/26075487




